NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) recommend testing for KRAS G12C in all eligible patients with advanced* NSCLC3,†,‡


NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) recommend testing for KRAS G12C in all eligible patients with advanced* NSCLC3,†,‡

CAP/IASLC/AMP and ASCO Guidelines recommend testing for actionable biomarkers (eg, KRAS G12C) utilizing either a comprehensive panel or targeted testing4

Support the ability of all NSCLC patients to obtain and understand their biomarker test results
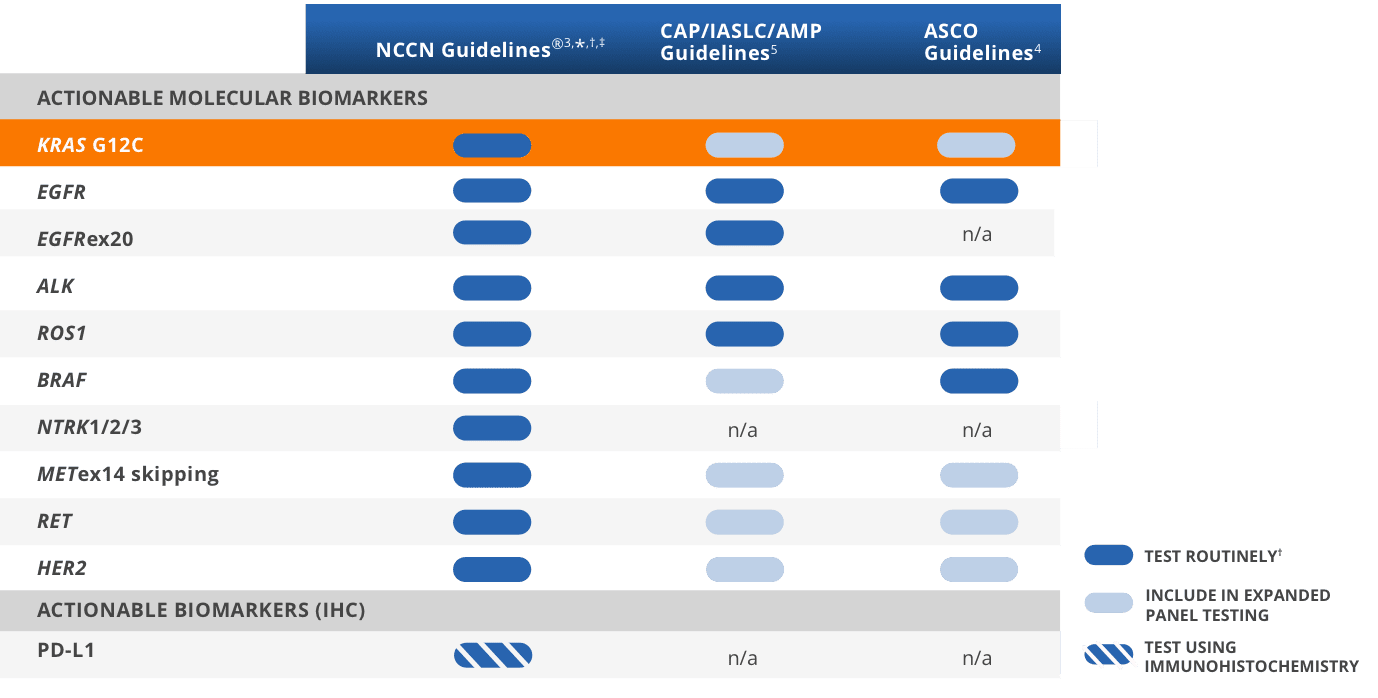
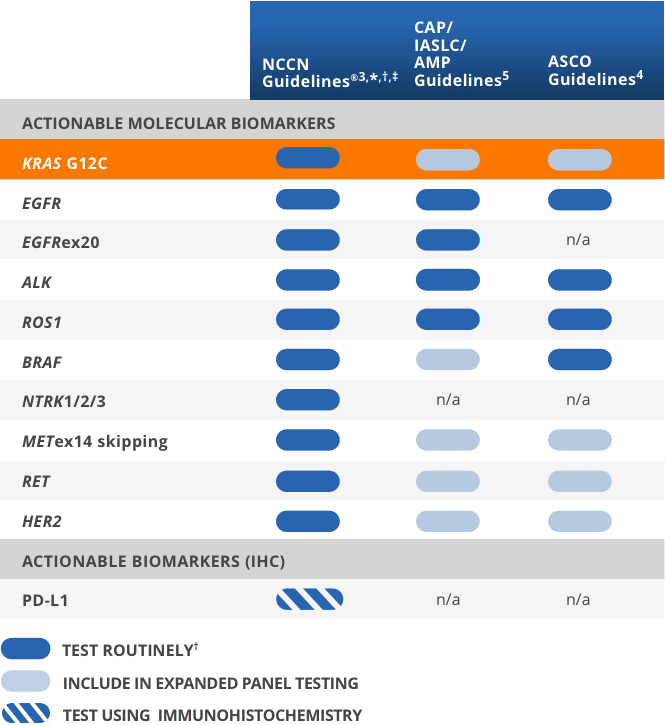
*Includes patients with advanced/metastatic NSCLC, but not locally advanced NSCLC.3
†It is recommended at this time that when feasible, testing be performed
via a broad, panel-based approach, most typically performed by NGS.3
‡The NCCN Guidelines® for NSCLC provide recommendations for individual biomarkers that should be tested and recommend testing techniques but do not endorse any specific
commercially available biomarker assays or commercial laboratories.3

A broad panel uses less tissue than a series of single-gene tests, so additional tests can be run if needed2

Broad-panel testing can be cost-effective based on the number of biomarkers requiring testing in NSCLC and may avoid the added cost and risk of rebiopsy6

Expanded-panel testing at diagnosis may take less time than consecutive single-gene tests in a process-of-elimination approach6

Expanded-panel testing provides results for many biomarkers in one report rather than different reports2

While the KRAS G12C mutation is most commonly found in smokers, it can occur regardless of clinical or demographic characteristics8

Guidelines recommend testing for actionable biomarkers including KRAS G12C in patients with advanced NSCLC2
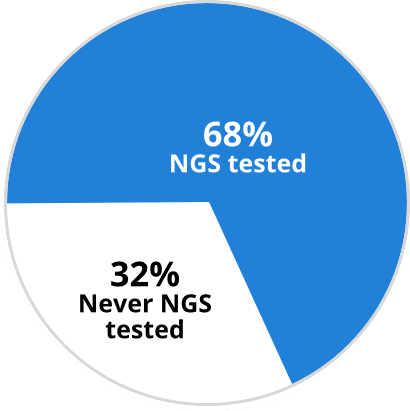
Percentage of patients receiving
comprehensive (NGS) biomarker testing (2020)9
The KRAS G12C mutation can be detected in tissue and liquid biopsy specimens using common molecular testing methods2
Most NGS platforms already test for KRAS G12C. There are also many PCR platforms capable of KRAS G12C testing7
| Test type | FDA-approved CDx for KRAS G12C patient identification |
|---|---|
| NGS |
|
| PCR | therascreen KRAS RGQ PCR kit (Qiagen Manchester, Ltd., tissue) |
Liquid biopsy may be used as an alternative to tissue-based testing14
Image reproduced with permission from Pantel K, et al.15
§The total amount of ctDNA may be < 0.01% of the total cfDNA concentration.15
One form of liquid biopsy consists of obtaining ctDNA from plasma, which can be used to identify genomic alterations15
Liquid biopsy can provide a comprehensive biomarker profile that may inform treatment decisions after 1L NSCLC therapy16,17
NSCLC liquid biopsy options exist19,20
Liquid biopsy can enable the identification of actionable biomarkers (such as KRAS G12C) and may inform treatment decisions following 1L NSCLC therapy16,17
**Concordance of PPV for the other guideline-recommended biomarkers was similarly high.14
††Among 282 patients with untreated non-squamous mNSCLC, the NILE study (Non-invasive versus Invasive Lung Evaluation) (N=307) enrolled patients at one of 28 North American centers between July 2016 and April 2018. The number of patients identified to have a driver mutation increased from 60 to 89, including those with negative, not assessed for the biomarker identified in cfDNA, or insufficient tissue results. The guideline-recommended biomarkers in this study include: EGFR, ALK, ROS1, BRAF V600E, RET fusions, METex14 skipping, MET amplification, KRAS mutations, and ERBB2 (HER2). The number of patients who received complete genotyping were 268 cfDNA vs 51 tissue (P < 0.0001). Turnaround time was defined as the number of days between test order date and the retrieval of test results.14
Concordance between ctDNA testing and tissue testing22,‡‡
If a liquid biopsy returns a positive result, it is actionable. If results are negative for actionable biomarkers, it is recommended to perform a tissue test as the presence of driver mutations cannot be ruled out from a liquid biopsy sample alone16,18
‡‡Concordance measured using 109 matched plasma and tissue samples from the Amgen 20170543 study. Samples were evaluated for KRAS G12C-positive or -negative status using the therascreen KRAS RGQ PCR test for tissue and Guardant360 CDx for cfDNA.22
Comparing Tissue vs Liquid Biopsy NGS Testing
††Among 282 patients with untreated non-squamous mNSCLC, the NILE study (Non-invasive versus Invasive Lung Evaluation) (N=307) enrolled patients at one of 28 North American centers between July 2016 and April 2018. The number of patients identified to have a driver mutation increased from 60 to 89, including those with negative, not assessed for the biomarker identified in cfDNA, or insufficient tissue results. The guideline-recommended biomarkers in this study include: EGFR, ALK, ROS1, BRAF V600E, RET fusions, METex14 skipping, MET amplification, KRAS mutations, and ERBB2 (HER2). The number of patients who received complete genotyping were 268 cfDNA vs 51 tissue (P < 0.0001). Turnaround time was defined as the number of days between test order date and the retrieval of test results.14
§§FDA-approved targeted therapies included EGFR, ALK, ROS1, BRAF V600E, RET, MET exon 14, ERBB2 (HER2), and KRAS14,16
Considerations that your multidisciplinary team (MDT) may find helpful when reporting biomarker results
Your MDT may include oncologists, pathologists, thoracic surgeons, pulmonologists, and others18
Consider using the upfront summary of your reporting template to document actionable biomarkers13,27
Consider uniform and unambiguous nomenclature (ie, KRAS G12C)13
Consider recording patients’ biomarker status in your notes or their chart for ease of future reference29
Consider using a set location for reports for easy access by all members of the MDT29
Check with your current pathologist or lab to confirm KRAS G12C is already included in their panel for NSCLC. However, if testing needs to be sent out, see below for a list of some national reference labs that offer testing for KRAS G12C
| Lab/Location | Phone Number/Website | ||
|---|---|---|---|
| Lab | Location | Website | Phone Number |
| ARUP LaboratoriesARUP Laboratories Salt Lake City, UT |
Salt Lake City, UT | https://www.aruplab.com | (800) 522-2787 |
| https://www.aruplab.com | |||
| BioceptBiocept San Diego, CA |
San Diego, CA | https://biocept.com | (888) 332-7729 |
| https://biocept.com | |||
| BiodesixBiodesix Boulder, CO |
Boulder, CO | https://www.biodesix.com | (303) 417-0500 |
| https://www.biodesix.com | |||
| Caris Life SciencesCaris Life Sciences Phoenix, AZ |
Phoenix, AZ | https://www.carislifesciences.com | (888) 979-8669 |
| https://www.carislifesciences.com | |||
| Circulogene TheranosticsCirculogene Theranostics Birmingham, AL |
Birmingham, AL | https://circulogene.com | (855) 614-7083 |
| https://circulogene.com | |||
| Foundation MedicineFoundation Medicine Cambridge, MA |
Cambridge, MA | https://www.foundationmedicine.com | (888) 988-3639 |
| https://www.foundationmedicine.com | |||
| GenPath DiagnosticsGenPath Diagnostics Elmwood Park, NJ |
Elmwood Park, NJ | https://www.genpathdiagnostics.com | (800) 627-1479 |
| https://www.genpathdiagnostics.com | |||
| Guardant HealthGuardant Health Redwood City, CA |
Redwood City, CA | https://guardanthealth.com | (855) 698-8887 |
| https://guardanthealth.com | |||
| Integrated Oncology / LabCorp Integrated Oncology / LabCorp Phoenix, AZ |
Phoenix, AZ | https://oncology.labcorp.com/ |
AZ: (800) 710-1800 CT/NY: (800) 447-5816 TN: (800) 874-8532 |
| https://oncology.labcorp.com | |||
| Mayo Clinic LaboratoriesMayo Clinic Laboratories Rochester, MN |
Rochester, MN | https://www.mayocliniclabs.com |
(800) 533-1710 or (507) 266-5700 |
| https://www.mayocliniclabs.com | |||
| NeoGenomics LaboratoriesNeoGenomics Laboratories Fort Myers, FL |
Fort Myers, FL | https://neogenomics.com | (866) 776-5907 (option 3) |
| https://neogenomics.com | |||
| NovogeneNovogene Sacramento, CA |
Sacramento, CA | https://en.novogene.com | (916) 252-0068 |
| https://en.novogene.com | |||
| Paradigm Cancer DiagnosticsParadigm Cancer Diagnostics Phoenix, AZ |
Phoenix, AZ | https://www.paradigmdx.com | (844) 232-4719 |
| https://www.paradigmdx.com | |||
| PredicinePredicine Hayward, CA |
Hayward, CA | https://www.predicine.com | (650) 300-2188 |
| https://www.predicine.com | |||
| Quest DiagnosticsQuest Diagnostics Teterboro, NJ |
Teterboro, NJ | https://www.questdiagnostics.com | (866) 697-8378 |
| https://www.questdiagnostics.com | |||
| Strata OncologyStrata Oncology Ann Arbor, MI |
Ann Arbor, MI | https://www.strataoncology.com | (734) 527-1000 |
| https://www.strataoncology.com | |||
| TempusTempus Chicago, IL |
Chicago, IL | https://www.tempus.com | (800) 739-4137 |
| https://www.tempus.com | |||
| TriCore Reference LaboratoriesTriCore Reference Laboratories Albuquerque, NM |
Albuquerque, NM | https://www.tricore.org/home |
(505) 938-8888 or (800) 245-3296 |
| https://www.tricore.org/home | |||
The above is a list of facilities with high testing volume that are CLIA certified and accept external samples. CLIA certification was validated using the CDC website, and acceptance of external samples was confirmed by reviewing facility websites and/or contacting facilities directly. Amgen neither recommends nor endorses, and may or may not have financial relationships with, any facility that appears on this list. This list is not intended to be a comprehensive list nor as a referral to any lab listed. If you would like to suggest a facility to be added to this list, please contact Amgen MedInfo at 800-77-AMGEN.
This information is current as of September 9, 2021. Amgen does not guarantee the accuracy of this information, and it is up to the individual healthcare professional to conduct his/her own research.
1L, first line; ALK, anaplastic lymphoma kinase; AMP, Association for Molecular Pathology; ASCO, American Society of Clinical Oncology; BRAF, proto-oncogene B-Raf; CAP, College of American Pathologists; CDC, Centers for Disease Control and Prevention; CDx, companion diagnostic; cfDNA, circulating free DNA; CI, confidence interval; CLIA, Clinical Laboratory Improvement Amendments; CTC, circulating tumor cells; ctDNA, circulating tumor DNA; EGFR, epidermal growth factor receptor; EMR, electronic medical record; ERBB2, erb-B2 receptor tyrosine kinase 2; FFPE, formalin-fixed, paraffin-embedded; GGT, glycine; HER2, human epidermal growth factor receptor 2; IASLC, International Association for the Study of Lung Cancer; IHC, immunohistochemistry; KRAS, Kirsten rat sarcoma; MET, mesenchymal-to-epithelial transition; mNSCLC, metastatic non-small cell lung cancer; n/a, not applicable; NCCN, National Comprehensive Cancer Network; NGS, next-generation sequencing; NSCLC, non-small cell lung cancer; NTRK, neurotrophic tyrosine receptor kinase; PCR, polymerase chain reaction; PD-L1, programmed cell death ligand 1; PPV, positive predictive value; RET, rearranged during transfection; ROS1, c-ros oncogene 1; TGT, cysteine; TSA, tumor surface area.